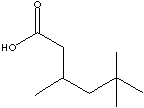
|
3,5,5-TRIMETHYL HEXANOIC ACID | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
3302-10-1 |
|
| EINECS NO. | 221-975-0 | |
| FORMULA | CH3C(CH3)2CH2CH(CH3)CH2COOH | |
| MOL WT. | 158.24 | |
| H.S. CODE | 2915.90 | |
|
TOXICITY |
Oral rat LD50: 3135 mg/kg | |
| SYNONYMS |
Isononanoic acid; 3,5,5-Trimethylhexansäure (German); | |
| ácido 3,5,5-trimetilhexanoico (Spanish); Acide 3,5,5-trimethylhexanoïque (French); | ||
| SMILES |
|
|
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
clear liquid | |
| MELTING POINT | ||
| BOILING POINT |
235 C | |
| SPECIFIC GRAVITY |
0.899 | |
| SOLUBILITY IN WATER |
sparingly soluble | |
| pH | 3.5 | |
| VAPOR DENSITY | ||
| AUTOIGNITION |
405 C | |
| NFPA RATINGS | Health: 2 Flammability: 1 Reactivity: 0 | |
| REFRACTIVE INDEX |
1.4278 -1.4298 | |
| FLASH POINT |
120 C | |
| STABILITY | Stable under ordinary conditions | |
|
DESCRIPTION AND APPLICATIONS |
||
|
3,5,5-Trimethylhexanoic Acid is used for modifying alkyd resins to prevent discolor and to keep flexibility and resistance to aging. It is used as a chemical intermediate. Its derivatives, acyl halides, anhydrides, esters, amides and nitriles, are used in making target products such as flavoring agents, pesticides, cosmetic ingredients, dyes, textile treatment agents, fungicides, and pharmaceuticals through further reactions of substitution, catalytic reduction, metal hydride reduction, diborane reduction, keto formation with organometallic reagents, electrophile bonding at oxygen, and Claisen condensation on carboxylic group. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
clear liquid | |
|
CONTENT |
90% min | |
|
NONANOIC ACIDS |
99.0% min | |
|
WATER |
0.1% max | |
| COLOR |
20 max (Pt/Co scale) | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 24/25 | ||
| GENERAL DESCRIPTION OF CARBOXYLIC ACID | ||
| Carboxylic acid is an organic compound whose molecules contain carboxyl group and have the condensed chemical formula R-C(=O)-OH in which a carbon atom is bonded to an oxygen atom by a solid bond and to a hydroxyl group by a single bond), where R is a hydrogen atom, an alkyl group, or an aryl group. Carboxylic acids can be synthesized if aldehyde is oxidized. Aldehyde can be obtained by oxidation of primary alcohol. Accordingly, carboxylic acid can be obtained by complete oxidation of primary alcohol. A variety of Carboxylic acids are abundant in nature and many carboxylic acids have their own trivial names. Examples are shown in table. In substitutive nomenclature, their names are formed by adding -oic acid' as the suffix to the name of the parent compound. The first character of carboxylic acid is acidity due to dissociation into H+ cations and RCOO- anions in aqueous solution. The two oxygen atoms are electronegatively charged and the hydrogen of a carboxyl group can be easily removed. The presence of electronegative groups next to the carboxylic group increases the acidity. For example, trichloroacetic acid is a stronger acid than acetic acid. Carboxylic acid is useful as a parent material to prepare many chemical derivatives due to the weak acidity of the hydroxyl hydrogen or due to the difference in electronegativity between carbon and oxygen. The easy dissociation of the hydroxyl oxygen-hydrogen provide reactions to form an ester with an alcohol and to form a water-soluble salt with an alkali. Almost infinite esters are formed through condensation reaction called esterification between carboxylic acid and alcohol, which produces water. The second reaction theory is the addition of electrons to the electron-deficient carbon atom of the carboxyl group. One more theory is decarboxylation (removal of carbon dioxide form carboxyl group). Carboxylic acids are used to synthesize acyl halides and acid anhydrides which are generally not target compounds. They are used as intermediates for the synthesis esters and amides, important derivatives from carboxylic acid in biochemistry as well as in industrial fields. There are almost infinite esters obtained from carboxylic acids. Esters are formed by removal of water from an acid and an alcohol. Carboxylic acid esters are used as in a variety of direct and indirect applications. Lower chain esters are used as flavouring base materials, plasticizers, solvent carriers and coupling agents. Higher chain compounds are used as components in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, wetting agents textile treatments and emollients, They are also used as intermediates for the manufacture of a variety of target compounds. The almost infinite esters provide a wide range of viscosity, specific gravity, vapor pressure, boiling point, and other physical and chemical properties for the proper application selections. Amides are formed from the reaction of a carboxylic acids with an amine. Carboxylic acid's reaction to link amino acids is wide in nature to form proteins (amide), the principal constituents of the protoplasm of all cells. Polyamide is a polymer containing repeated amide groups such as various kinds of nylon and polyacrylamides. Carboxylic acid are in our lives. | ||